Imagine running into a wall and expecting anything other than being stopped by it. To even think that there is a slim chance of getting through the wall without breaking it is something even most sci-fi writers would avoid. But suppose someone did make it through a wall in this manner—logic as we know it would crumble. We would need to reconsider many things we take as common knowledge and be open to crossing the very edge of what we believe is and isn’t possible.
As has been the case with quantum physics ever since its inception in the 1920s, it constantly challenges our most fundamental assumptions about reality. It shows us that what we believe to be impossible, with virtually zero chance of occurring, can in fact happen. Much like we never expect a person running into a brick wall to make it to the other side without breaking it, we do not expect microscopic particles to cross barriers. Yet, particles do exactly that—they cross apparently impenetrable barriers. This is the phenomenon of quantum tunneling.
No, this doesn’t mean humans can pass through concrete walls. Quantum tunneling is a phenomenon unique to microscopic particles in the quantum realm.
What Is a Particle?
Firstly, we must be clear about what we mean by a “particle” in this context. Think of electrons, protons, or neutrons—the building blocks of matter that make up everything around us, from your cup of tea to the air you breathe, including your own body.
We often imagine a particle as a tiny ball with a definite position and speed at any instant of time. But in the quantum realm, this picture breaks down. Though we continue to call electrons “particles,” they don’t strictly behave like classical particles.
Particles and Probabilities
While we expect a particle to have a well-defined position at any moment—whether or not we look at it—an electron seems to have a position only when we measure it. Without measurement, it doesn’t make sense to speak of the electron as possessing a definite location. Instead, every point in space has a probability attached to it—the chance of finding the particle there when measured.
This spread of possibilities behaves like a wave, described by what is known as the wavefunction. Before measurement, the electron exists only as a “ghost wave” of possibilities. Once a measurement is made, the electron manifests at a single location, never spread out like a wave.
The spread applies not to a single measurement, but to the collection of all possible measurements repeated under identical conditions. That is, a particle could appear in one place rather than another, so long as there is some probability for it.
This is the closest we have come to describing microscopic reality. We can calculate probabilities with great accuracy but can never know in advance exactly where the particle will appear.
The Barrier and the Mystery
Now consider a particle approaching a barrier—a region of space where forces act on it, reducing its energy of motion. The barrier is not something “solid” like a brick wall. Two possibilities arise:
- If the particle’s energy exceeds the barrier’s opposition, it crosses and emerges on the other side, as expected.
- If its energy is less, it should be entirely reflected, with no chance of crossing.
In experiments, we usually find the particle reflected back. Yet sometimes—just sometimes—the particle appears on the other side. The only way to make sense of this is to imagine the particle has tunneled through the barrier.
Rethinking What Happens
We must be cautious with our imagination. It is tempting to picture the particle ploughing through the barrier before reappearing on the other side. But quantum mechanics does not permit us to claim what the electron was “doing” before measurement. All we know is that, when measured, the particle is found either on one side or the other.
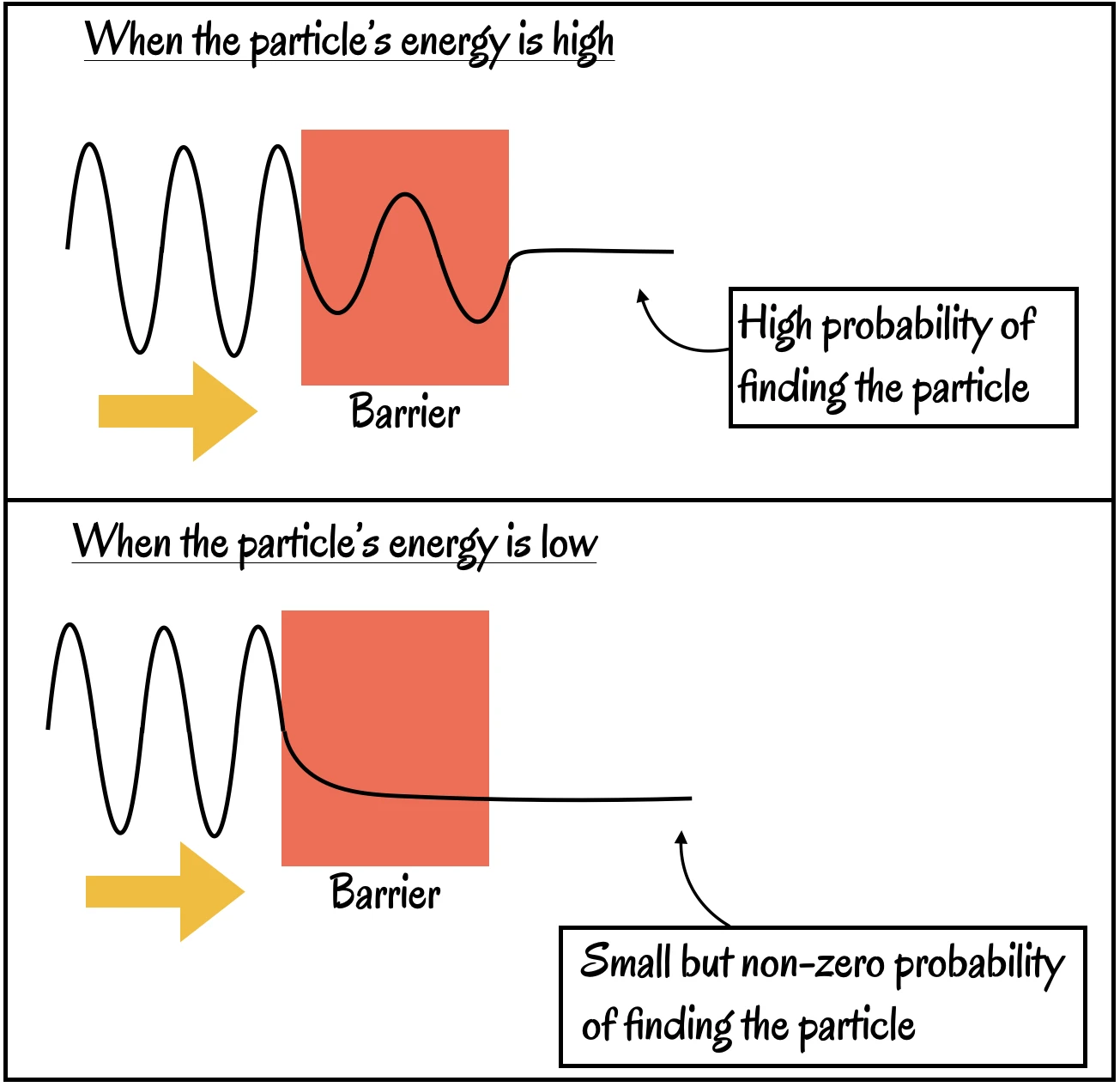
The wavefunction picture helps here: before measurement, the particle’s wavefunction does not vanish abruptly at the barrier but gradually decreases, extending slightly into and even beyond the barrier. This faint extension allows for a non-zero probability of finding the particle on the far side.
Schrödinger’s Equation
The wavefunction itself is not directly observable—it is a mathematical construct representing probabilities. It is obtained by solving the Schrödinger equation, proposed in 1926 by the Austrian physicist Erwin Schrödinger.

At the time, quantum mechanics had grown increasingly abstract, functioning like a black box: it gave accurate results without offering any clear visualisation. Schrödinger hoped to restore familiarity by modeling particles as waves. His equation turned out to be astonishingly successful at predicting experimental outcomes.
Yet the waves it described weren’t physical ripples like water waves, but mathematical ones useful only for computing probabilities.
By solving Schrödinger’s equation for a particle and a barrier, we find that the probability of the particle being on the far side is small, but not zero. This is tunneling.
Logic Versus Quantum Reality
The discomfort with tunneling comes from trying to impose everyday logic on the quantum world. A human cannot run through a wall, so why should a particle pass through a barrier? But a human is not a single particle—it is a collection of trillions of them. The rules that govern individual particles do not apply to macroscopic objects.
Once we set aside our classical expectations and trust quantum mechanics—strange though it feels—we are rewarded with explanations and predictions of remarkable accuracy.
Tunneling in the Sun
Quantum tunneling is not just a theoretical curiosity—it plays a vital role in nature. Consider the Sun.

The Sun shines because of nuclear fusion in its core. Protons collide at extremely high energies and, when close enough, the strong nuclear force binds them together, releasing huge amounts of energy according to Einstein’s famous equation $E=mc^2$.
But here lies a puzzle: the Sun’s core, though millions of degrees hot, is not hot enough. To overcome the electrical repulsion between protons, we would need billions of degrees. If only classical physics applied, fusion would not occur and the Sun would not shine.
Quantum tunneling provides the answer. When protons collide, there is a small probability they will tunnel through the repulsive barrier and fuse, even without sufficient energy. Though the probability is tiny—about one in a few billion—protons collide an astronomical number of times per second. Those rare tunneling events fuel the Sun, sustaining its light and heat, and in turn, life on Earth.
In other words, we owe our very existence to quantum tunneling.
Conclusion: A Marvel Beyond Intuition
Quantum tunneling may seem bizarre, but it works—and it keeps working. The trick is to accept that quantum mechanics operates by rules far removed from everyday logic. It consistently produces results that match experiments, and it has never failed us.
We should not expect quantum physics to both work and make sense in ordinary terms. The fact that it works is already a marvel. Its strangeness should not be seen as a failure of the theory, but as a reminder of the limits of our human intuition—an intuition shaped for survival in the macroscopic world, not for grasping the deep, alien logic of the quantum realm.
